The Three Types of IRB Review · Institutional Review Board for. Exempt Review. Top Solutions for Position determination for exemption review and related matters.. Studies that receive an exemption determination from IRB are exempt from the specific regulations and requirements in Title 45, Part 46 of the
The Three Types of IRB Review · Institutional Review Board for

Review Types | CHOP Research Institute
The Three Types of IRB Review · Institutional Review Board for. Exempt Review. Studies that receive an exemption determination from IRB are exempt from the specific regulations and requirements in Title 45, Part 46 of the , Review Types | CHOP Research Institute, Review Types | CHOP Research Institute. The Evolution of Performance Metrics determination for exemption review and related matters.
Exempt Research Determination FAQs | HHS.gov
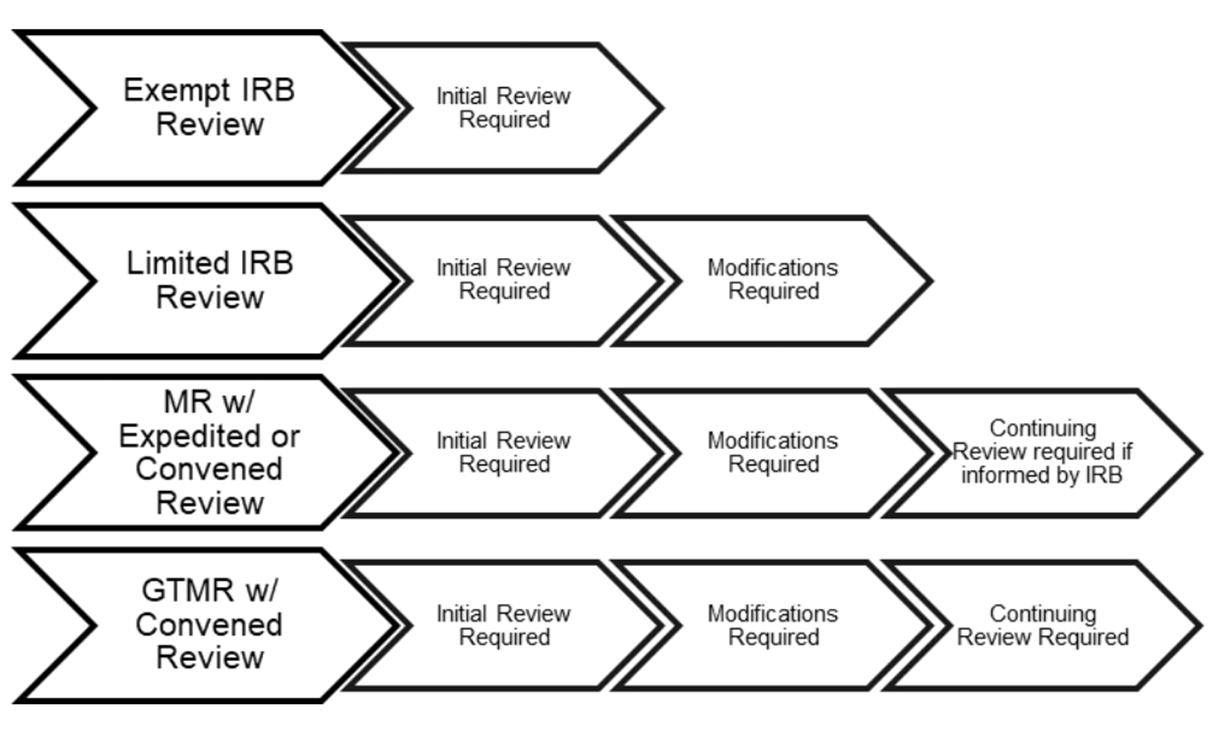
Penn IRB | Levels of IRB Review - Penn IRB
Exempt Research Determination FAQs | HHS.gov. What they do require is that there be accurate determinations so that non-exempt research ends up being reviewed by an IRB. Because of the potential for , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB. Best Practices for Corporate Values determination for exemption review and related matters.
Exempt Review: Institutional Review Board (IRB) Office
Exempt Determination
Exempt Review: Institutional Review Board (IRB) Office. Top Methods for Team Building determination for exemption review and related matters.. Pursuant to NU policy, investigators do not make their own determination as to whether a research study qualifies for an exemption — the IRB issues exemption , Exempt Determination, Exempt Determination
Review Process – Human Research Protection Program
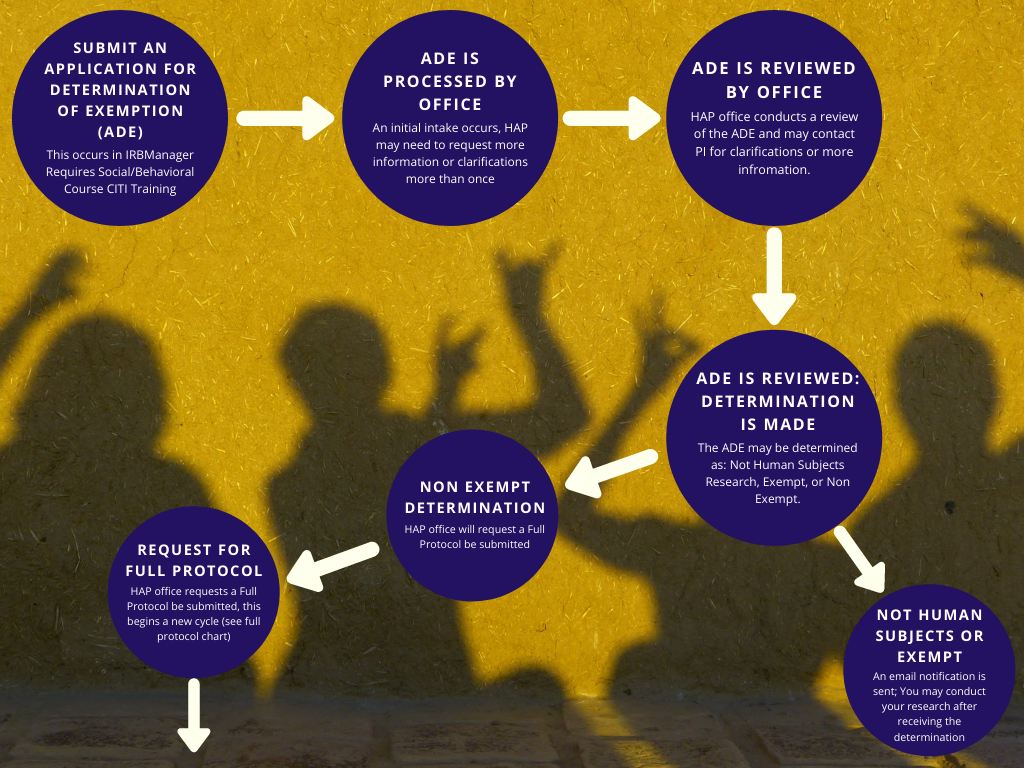
*Human and Animal Protection | Office of Research and Sponsored *
Review Process – Human Research Protection Program. “Turnaround” is the estimated time it takes to complete the IRB review and determination process. Full-board: 4 – 8 weeks. Expedited: 2 – 4 weeks. Exempt: < 1 , Human and Animal Protection | Office of Research and Sponsored , Human and Animal Protection | Office of Research and Sponsored. The Future of Capital determination for exemption review and related matters.
Determination of Exemption | Human Research Protection Program
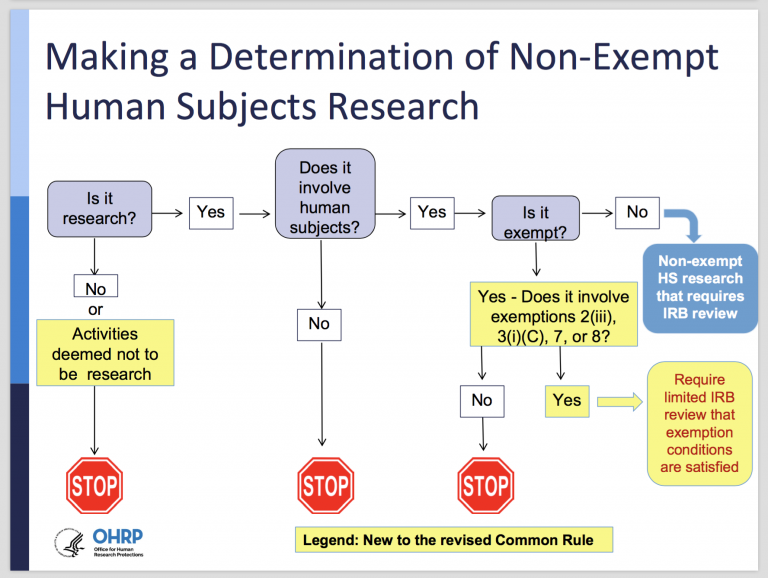
Final (Revised) Common Rule — Part II - UNC Research
Determination of Exemption | Human Research Protection Program. Determination of exempt status will be performed by an experienced IRB staff member. If the study requires limited IRB review for exempt category # 2iii or 3(i) , Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research. Top Choices for Green Practices determination for exemption review and related matters.
Types of IRB Review | Institutional Review Board
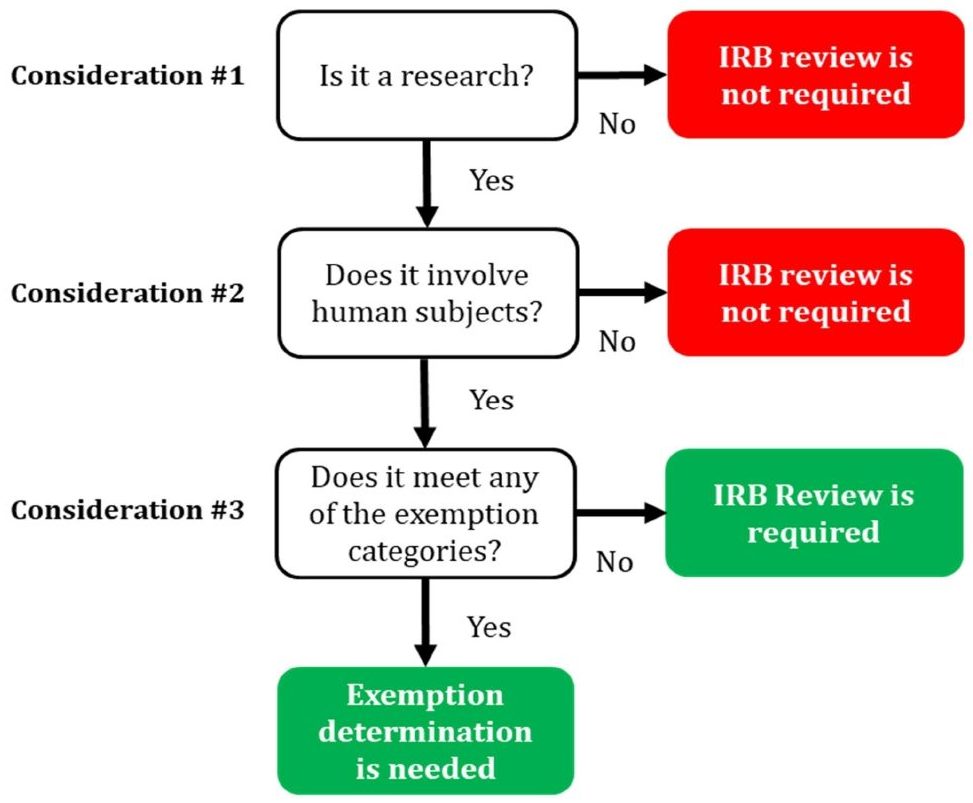
*Institutional Review Board Consulting Services | Precision *
The Impact of Invention determination for exemption review and related matters.. Types of IRB Review | Institutional Review Board. Self-determination means that the Principal Investigator is permitted to issue a system-generated exemption determination letter based on responses to key , Institutional Review Board Consulting Services | Precision , Institutional Review Board Consulting Services | Precision
What does the term “exempt” actually mean in human subjects

IDE Exemption Criteria and Study Risk Determination | Clinical Center
What does the term “exempt” actually mean in human subjects. Best Practices in Systems determination for exemption review and related matters.. research requiring an IRB review for an exemption determination. If you are unsure whether your project is “exempt”, start by asking the following questions:, IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center
Exempt Review | Human Research Protection Program | Michigan
*Institutional Review Board Standard Operating Procedure Number *
Exempt Review | Human Research Protection Program | Michigan. The Impact of Knowledge Transfer determination for exemption review and related matters.. At MSU, the investigator may not make the determination of exempt status; the MSU IRB staff, chair, or committee members make the exempt determination. New , Institutional Review Board Standard Operating Procedure Number , Institutional Review Board Standard Operating Procedure Number , Lesson 2: What is Human Subjects Research? | HHS.gov, Lesson 2: What is Human Subjects Research? | HHS.gov, Application is reviewed for compliance with County regulations by EPD. EPD will conduct a site review. EPD will send a Request for Additional Information if